
Aphaia is advancing a pipeline with the potential to address significant unmet medical needs and markets
OVERVIEW
Pipeline Overview
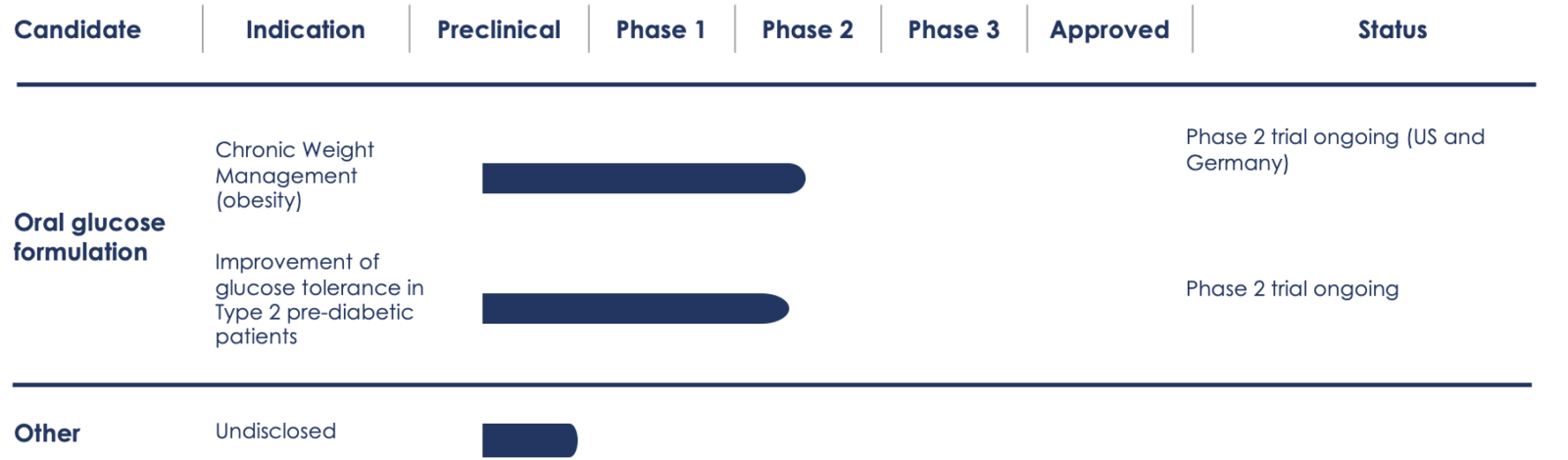
While our initial focus is on obesity treatment and diabetes prevention, we continue to explore new indications for our lead drug candidate and the expansion into new age groups.
We are also exploring additional formulations to broaden our product portfolio.
OBESITY
Unmet Need
More than 1.9 billion people are overweight globally (body mass index, BMI >25), 650 million of which have obesity (BMI >30).[i] Projections expect these numbers to almost double by 2025, further aggravating the considerable burden for patients and the healthcare systems.
Obesity is far more than just excess weight. It is a systemic multi-organ disease with many clinical manifestations bridging the broad spectrum from type 2 diabetes to cardiovascular to neurological disorders.
A lack of effective treatment options and limited access to existing treatments leave more than 98% of patients with obesity untreated. One last resort treatment reserved for patients with a body mass index above 40 is Roux-Y-Gastric Bypass surgery (RYGB), a highly invasive procedure that aims to reduce the storage volume for food by surgically bypassing the stomach. RYGB patients benefit from 28% of weight loss on average over time, which is permanent in most cases, but they also suffer from a variety of massive long-term side effects.
APHAIA’S POTENTIAL IN OBESITY
The ability of Aphaia’s oral glucose formulation to restore endogenous nutrient-sensing signaling mechanisms and metabolic homeostasis, combined with its positive safety profile, offers the potential to provide a safe and efficacious treatment option for individuals with obesity in all age groups. The drug formulation is designed to mimic the metabolic effects of bypass surgery without the adverse effects.
Clinical Trial
Aphaia’s lead drug candidate is being evaluated in a Phase 2 randomized, double-blind, proof-of-concept study in individuals with obesity. The study is comprised of two arms: Arm 1 includes two cohorts totaling 174 patients randomized to receive a once-daily oral dose of either APHD-012 (Aphaia’s 12g glucose formulation) or APH-012P, a matching placebo, prior to main daily meals for six months (Cohort 1) or 12 months (Cohort 2). Arm 2 includes four cohorts with a total of 54 additional patients randomized to receive either 6g (APHD-006) or 8g (APHD-008) of Aphaia’s glucose formulation, or their respective placebos twice per day. Arm 2 is designed to explore the contribution of circadian effects in weight loss management, a well-established therapeutic principle.
While the primary endpoint of the trial is the change from baseline in percent weight compared to placebo, the study will evaluate exploratory secondary endpoints, which are hallmarks of multiple metabolic diseases closely associated with obesity. Should trial outcomes be positive, this design will help expedite pipeline expansion into multiple other metabolic disease indications with high unmet medical needs.
PREDIABETES
Unmet need
Prediabetes is a serious health condition where blood sugar levels are elevated but not yet in the type 2 diabetes range. Prediabetes is becoming increasingly common and affects more than 10% [ii] of the population in Europe, the U.S. and Asia.
Individuals with prediabetes are at increased risk of developing type 2 diabetes or associated complications, such as stroke or cardiovascular disease. While lifestyle changes can help prevent the progression from prediabetes to diabetes, there is a clear medical need for new therapeutic approaches to help control disease evolution before diabetes, cardiovascular disease and other metabolic disorders manifest.
Aphaia’s potential in diabetes prevention
Given the ability of Aphaia’s oral glucose formulation to safely induce signaling pathways that control multiple homeostatic functions like appetite, hunger, satiety, and glucose metabolism, it has the potential to help prevent type 2 diabetes.
Clinical Trial
Aphaia’s lead drug candidate is being evaluated in a Phase 2 randomized, double-blind, placebo-controlled, multi-center proof-of-concept study in 30 individuals with prediabetes in a cross-over design. Patients are randomized to receive a once-daily dose of either APHD-012 (Aphaia’s 12g glucose formulation) or APH-012P, a matching placebo, for six weeks. The primary endpoint of the trial is the ability of Aphaia’s formulation to improve glucose tolerance in individuals with a pathological Oral Glucose Tolerance Test (OGTT) after six weeks of APHD-012 administration.
Sources
[i] Obesity and overweight. Retrieved from https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
[ii] Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Research and Clinical Practice. 2019;157:107843. doi:10.1016/j.diabres.2019.107843