
Our goal is to restore metabolic balance to prevent and treat metabolic diseases
Many metabolic disorders, such as obesity or type 2 diabetes, are associated with disruptions in the gut’s natural endocrine, neuroendocrine and neuronal responses to food intake, which are crucial to maintaining a healthy metabolism. This disruption can arise from factors such as an imbalanced diet, sedentary lifestyle, genetic predispositions, or environmental factors.
Conventional obesity treatments such as incretin agonists, most notably GLP-1, adopt a hormone replacement strategy. This approach only partially addresses the root causes of these disorders by emulating the actions of hormones involved in hunger and glucose metabolism. Also, they often trigger side effects that may limit treatment eligibility or long-term use, as they elevate plasma hormone levels beyond natural physiological responses.
OUR SOLUTION – RESTORING METABOLIC BALANCE
Our goal is to restore a normal metabolic food response through natural and safe treatment alternatives that can be applied to broad populations and enable long-term use.
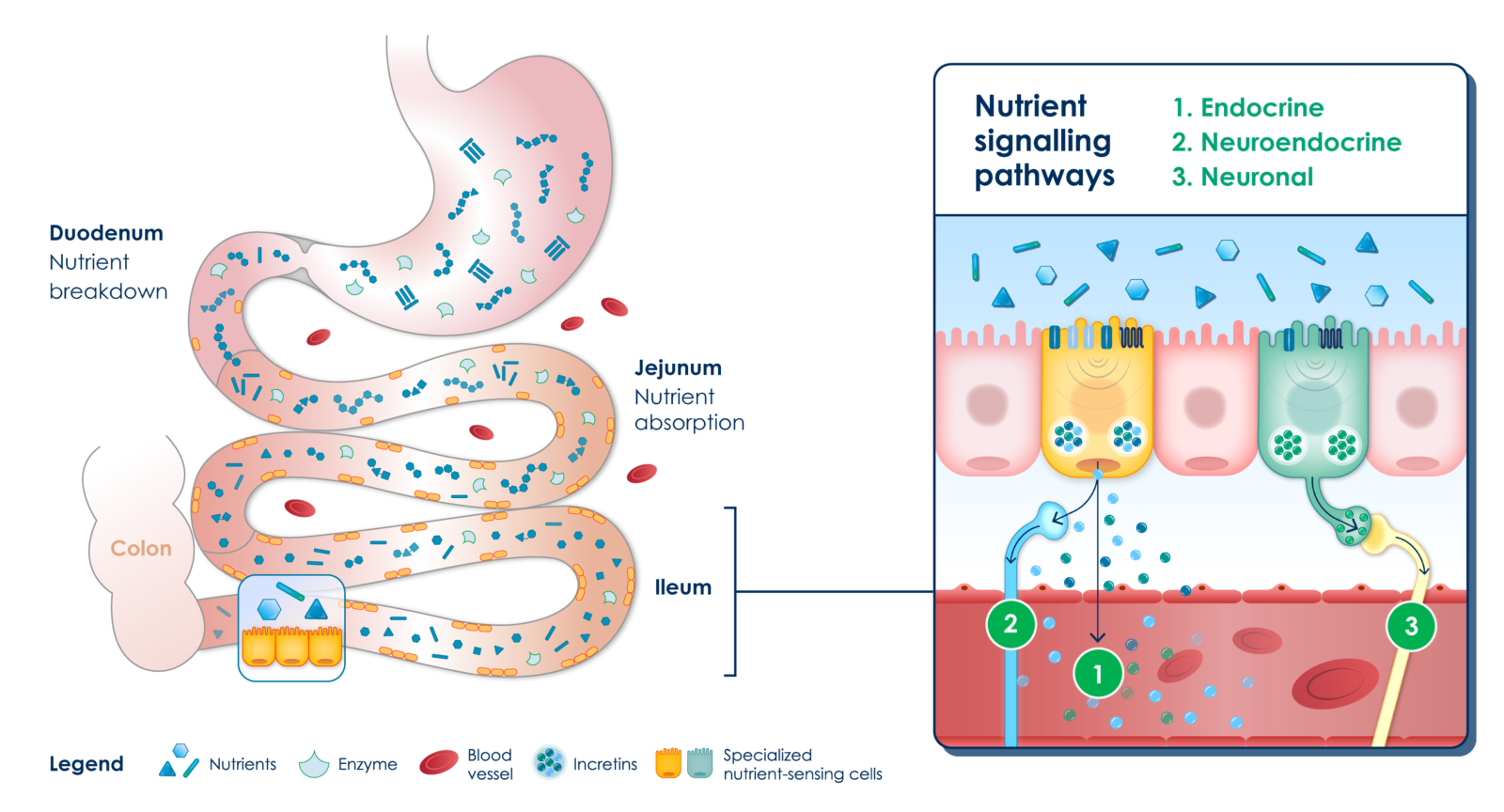
Healthy individuals have a balanced absorption of nutrients in the intestine. Upon food intake, the excess of nutrients not absorbed in the upper parts of the small intestine (i.e., duodenum and proximal jejunum) stimulates nutrient-sensing cells and their broad repertoire of signaling mechanisms (i.e., endocrine, neuroendocrine and neuronal) in the distal parts of the small intestine (i.e., distal jejunum and ileum). The broad portfolio of mechanisms, including the release of enteral hormones known as incretins, regulates various homeostatic functions like appetite and metabolic activity.
Healthy enteroendocrine system
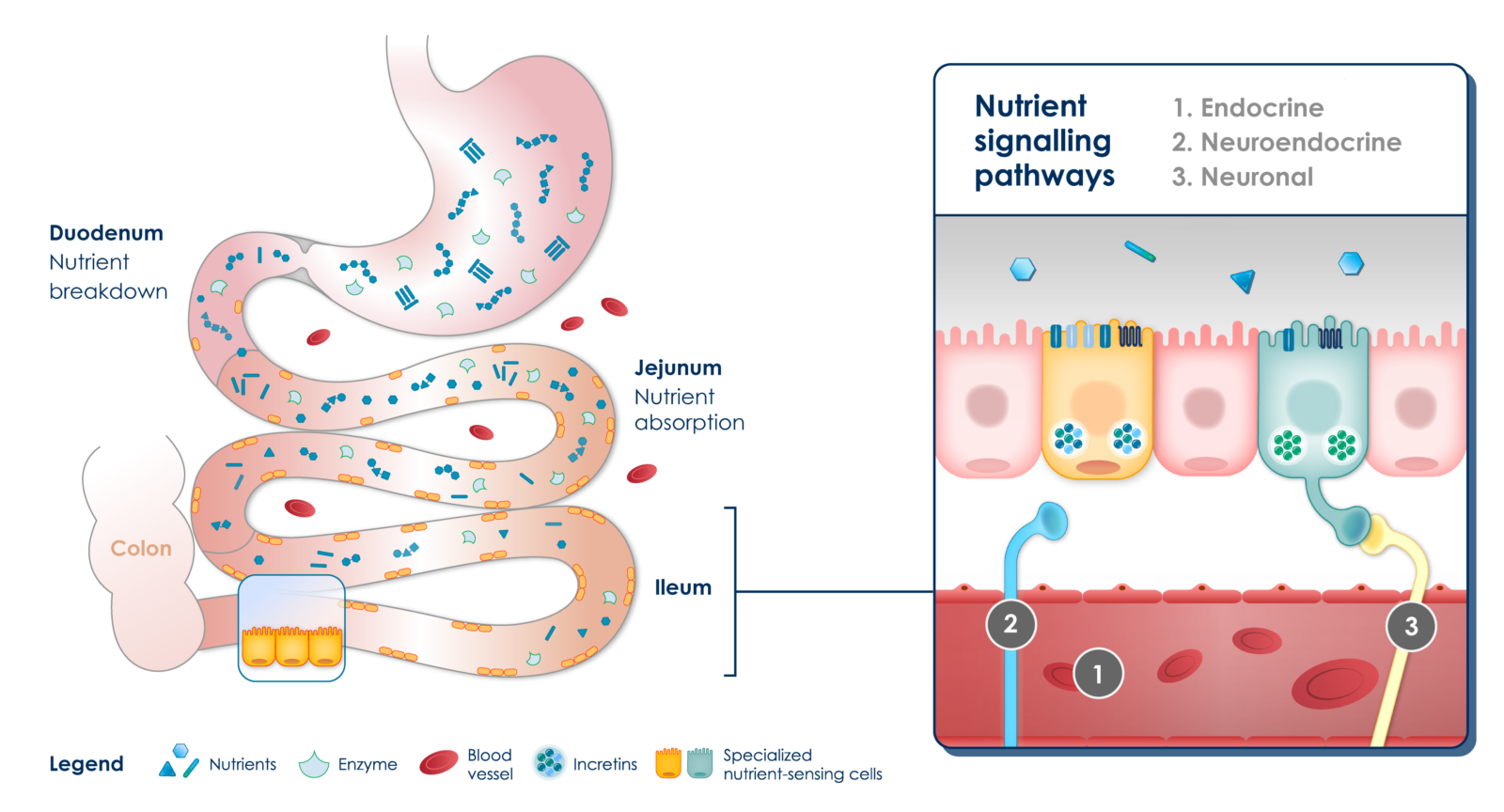
In patients with obesity and associated metabolic disorders, there is nearly complete absorption of nutrients in the upper parts of the small intestine after food intake. This absorption is promoted by the highly refined nature of food and its slow passage through the digestive tract. Consequently, distal nutrient-sensing cells are largely deprived of direct contact with food, resulting in a lack of endocrine, neuroendocrine and neuronal stimulation. This disruption of metabolic homeostasis contributes to the progression of metabolic diseases.
Reduced enteroendocrine responses
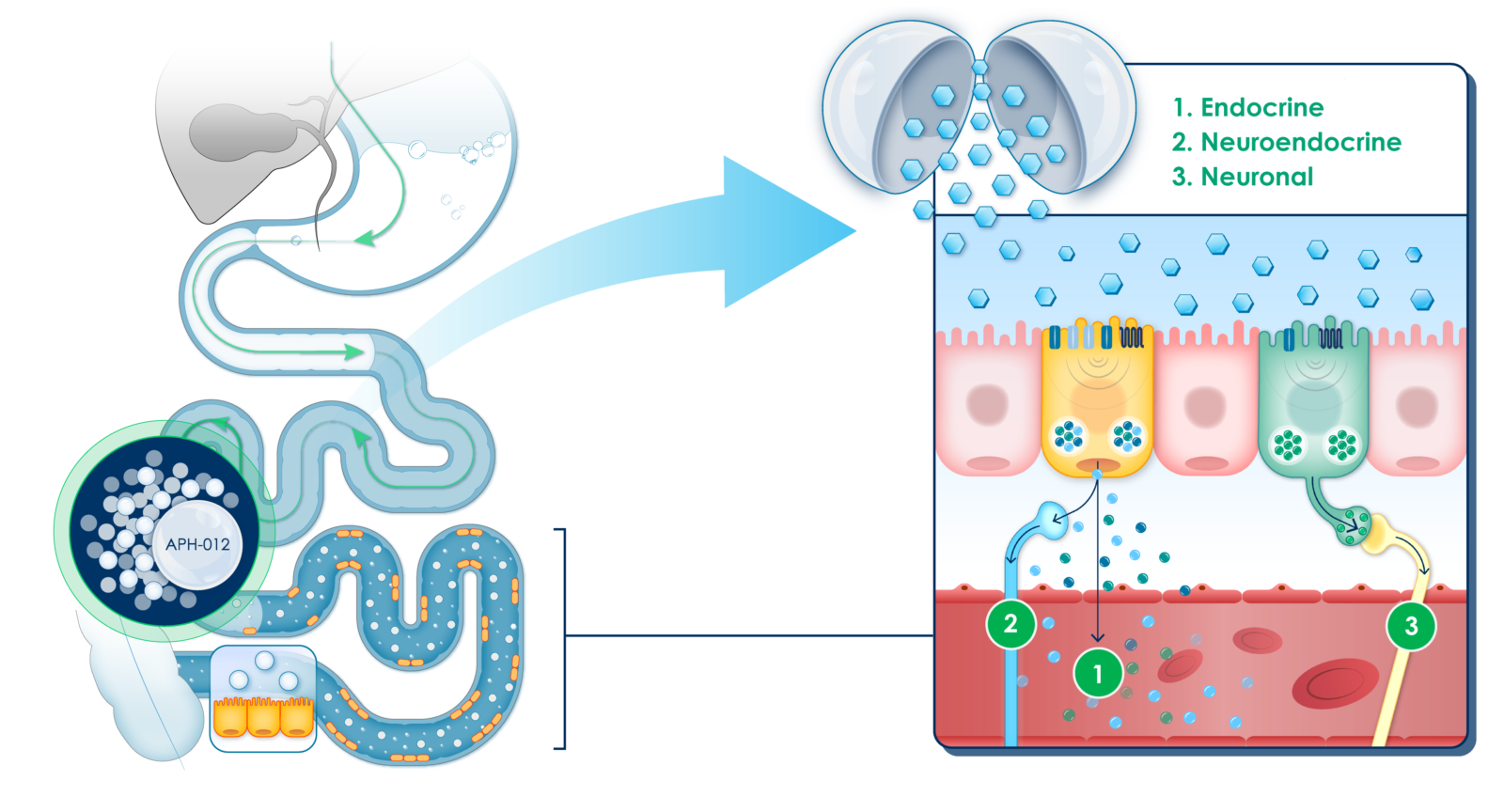
APHAIA’S LEAD CANDIDATE
Aphaia’s lead drug candidate is a proprietary oral formulation of coated glucose beads. It is designed to be released at discrete parts of the small intestine to reawaken nutrient-sensing cells that become less responsive in obesity and restore endocrine, neuroendocrine and neuronal signaling pathways.
By design of the beads, the glucose released from the formulation is not absorbed in the upper parts of the small intestine and is not systemically available, which is a key safety feature in general, but particularly in diabetic patients.
Aphaia selected glucose as its drug formulation for two key reasons. Firstly, its natural composition suggests a safer profile. Secondly, glucose maximizes nutrient-sensing cell responses, whereas other compounds may trigger insufficient or delayed hormone releases.
Mechanism of Action
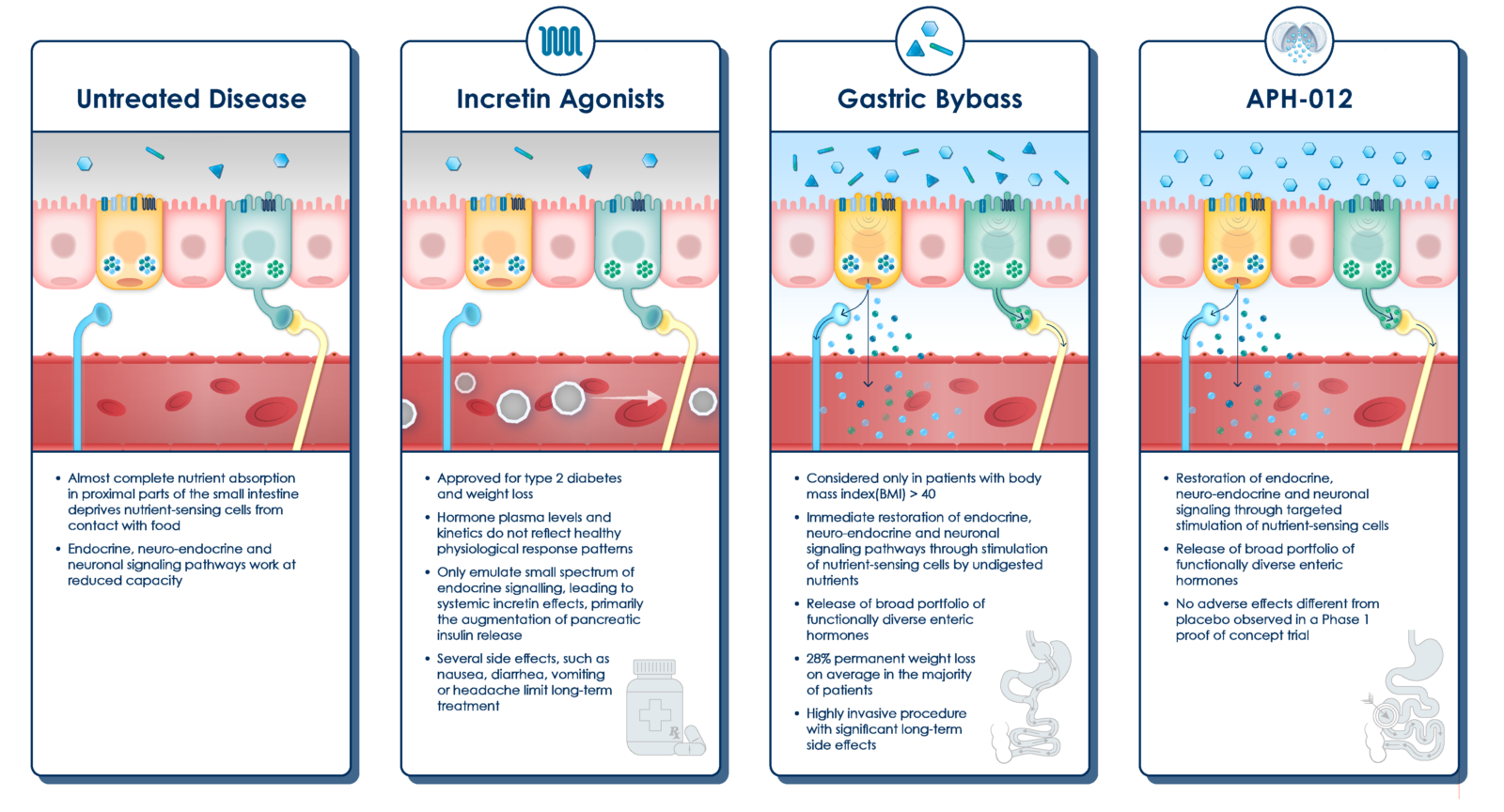
POTENTIAL OF APHAIA’S GLUCOSE FORMULATION
In a Phase 1 trial in individuals with obesity, targeted release of glucose with Aphaia’s proprietary formulation induced therapeutically relevant release of a broad spectrum of enteric hormones, including glucagon-like-peptide 1 (GLP-1), peptide tyrosine-tyrosine (PYY), glicentin and oxyntomodulin (OXM) among others.
This effect has not previously been achieved with any other drug-based therapy since hormone-based therapies, such as GLP-1 or dual agonists, only partially correct the hormone deficiency. They also necessarily induce unphysiological serum concentration peaks of hormones that lead to side effects that limit long-term use [i].
The levels of hormones released by the enteroendocrine cell, upon stimulation by the glucose formulation, were comparable to those measured after ROUX-Y Gastric Bypass surgery (RYGB). RYGB is the only treatment solution at present that addresses the underlying condition of obesity, but it has massive side effects that negatively impact the quality of life, well-being and prognosis.
In the Phase 1 trial, treatment with Aphaia’s oral glucose formulation did not induce any adverse events that were different from those in the placebo group, and as such, it has the potential to mimic the metabolic effects of bypass surgery without adverse effects.
Competitive Advantage
APHAIA’S PRECISION-TARGETED DRUG FORMULATIONS WITH BROAD POTENTIAL
Beyond its lead candidate, Aphaia’s platform and approach enables the development of a variety of proprietary drug formulations for targeted delivery of natural compounds to discrete parts of the digestive tract.
- Natural and targeted: Coated bead formulations are designed to transport natural substances and release their cargo at pre-determined discrete parts of the small intestine. The strict limitation to natural substances as cargo should translate into excellent safety profiles.
- Reproducible and reliable: Coated beads enable highly reproducible transit patterns with low inter-individual variability, translating into treatment reproducibility and reliability (has already been demonstrated with APHD-012).
- Versatile: Coated beads are designed to enable variations in cargo formulations and target areas of the intestine, with the potential to address multiple indications.
Sources
[i] Chou C-Y, Chang Y-T, Yang J-L, et al. Effect of long-term incretin-based therapies on ischemic heart diseases in patients with type 2 diabetes mellitus: A network meta-analysis. Scientific Reports. 2017;7(1). doi:10.1038/s41598-017-16101-1